
Needlefree Access Devices
Request Samples »
Meet the Lyka® PORT Needlefree Access Device
For Hemodialysis Applications
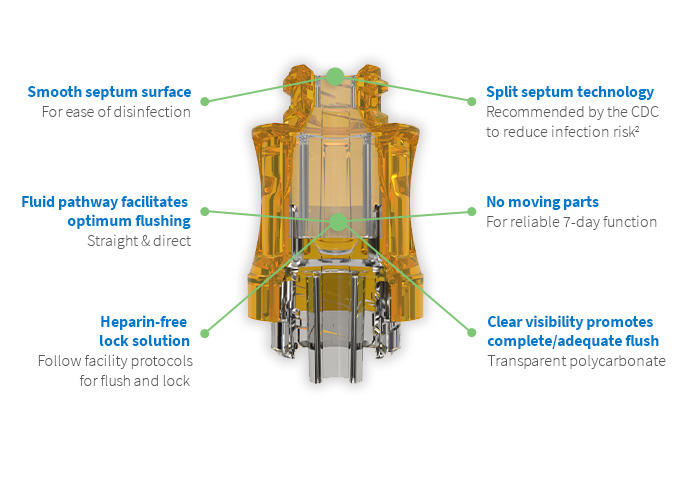
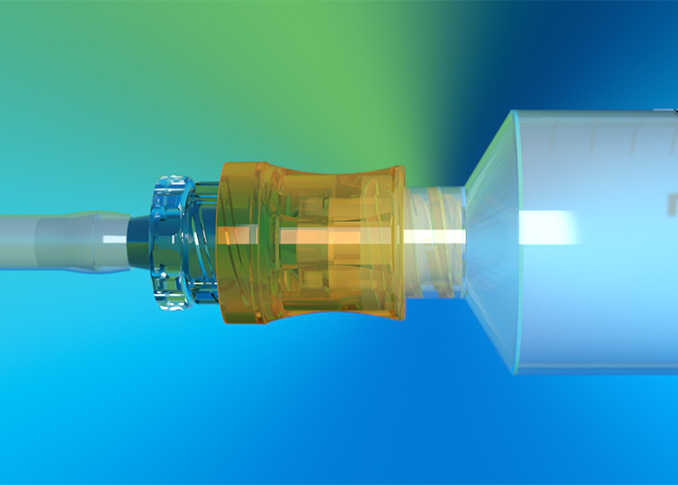
Uniquely designed for use in hemodialysis, the Lyka® PORT1 brings engineering excellence to patient care, safety, and performance. The Lyka® PORT needlefree access device utilizes split septum technology with low dead space, and is fully flushable to reduce infection risk. The CDC recommends split septum valves over mechanical valves in needleless systems to reduce infection risk. The Lyka® PORT features an open fluid path, delivering ultra-high flow rates and enhanced safety for hemodialysis patients2.
Needlefree Connectors
Nordson MEDICAL revolutionizes split septum technology with enhanced safety features that assist in reducing infection control rates. Designed for a range of applications, our needlefree connectors enable multiple activations and maintain a sterile, closed system for increased patient safety and improved outcomes. Browse our product catalog and request product samples today!
Enlarge Image
Engineering Excellence for Hemodialysis
Features & Benefits
- High flow rate of up to 600 mL/min for efficient hemodialysis3
- Straight fluid path, low dead space, and fully flushable
- Translucent housing for visual check
- Precise split septum technology with low dead space provides an effective microbiological barrier5
- Approved for 7-day use4
Validated for Hemodialysis Duration
Lyka® PORT Advantages
The Lyka® PORT needlefree access device is validated for use as a physical and microbial barrier for up to 7 days, eliminating the need for replacement after every treatment. The Lyka® PORT's long duration of use represents a critical advantage for hemodialysis patients who require consistent, long-term vascular access.
Minimizing Risk
Reducing Catheter-Related Blood Stream Infections
Catheter-related bloodstream infections, occlusion, and leakage are common complications among hemodialysis patients with central venous catheters. The Lyka® PORT needlefree access device is designed to minimize these risks and improve patient safety.
Improving Dialysis Care
Provide a safe and effective solution for patient care during hemodialysis treatments with the Lyka® PORT1. To learn more about the newest innovation in dialysis care, read our white paper, 'Lyka® PORT Needlefree Access Device for Hemodialysis: a New Standard in Patient Safety and Performance'.
References
1 Lyka® PORT is FDA 510(k) cleared for use with vascular access devices in hemodialysis or as an accessory to an I.V. set for the administration or withdraw of fluids to a patient through a cannula or needle placed in the vein or artery. The device may be used for patient populations including very low birth-weight infants, infants, children, and adults for up to 7 days
2 O-Grady, Naomi, et al. “Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011.” U.S. Centers for Disease Control and Prevention, 2011, pp. 19
3 Data on file
4 Data on file
5 Data on file
For complete indications, contraindications, warnings, precautions, and potential adverse events, refer to the product's Instructions for Use (IFU).
