Needlefree Connectors
Request Samples »
Innovative Q2® Technology
For Better Patient Care
We've revolutionized infusion sites with needle-free split septum technology, enhancing safety features that assist in reducing infection rates. Integrated into most of our infusion therapy products, the straight, unobstructed fluid path provides high flow rates and is engineered to suppress thrombotic occlusion risks caused by disconnecting syringes or secondary sets. Our needlefree connectors, now available for hemodialysis applications, enable multiple activations and maintain a sterile, closed system for increased patient safety and improved outcomes.
Lyka® PORT Needlefree Access Device for Hemodialysis
Uniquely designed for use in hemodialysis, the Lyka® PORT1 brings engineering excellence to patient care, safety, and performance. The Lyka ® PORT needlefree access device utilizes split septum technology with low dead space, and is fully flushable to reduce infection risk. The CDC recommends split septum valves over mechanical valves in needleless systems to reduce infection risk. The Lyka® PORT features an open fluid path, delivering ultra-high flow rates and enhanced safety for hemodialysis patients2.
Enlarge Image
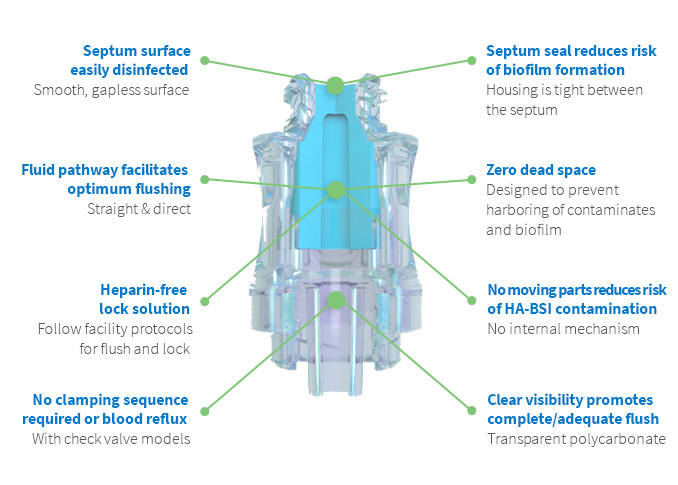
Benefits of Q2® Technology3
In Infusion Therapy Applications
Benefits of our innovative Q2® technology include:
- Reduced risk of HA-BSI contamination – no moving parts
- Low residual volume/dead space
- Easy disinfection with smooth septum surface
- Optimum flushing with a straight and direct fluid path
- Clear visibility for complete and adequate flush – transparent polycarbonate
Q2® Dual Port
T-Extension Set
Our extension sets focus on safe and accurate infusion therapy delivery with minimal priming volumes and high flow rates. Our offerings include various tubing sizes and component options to ensure your patients are kept safe.
References
1 Lyka® PORT is FDA 510(k) cleared for use with vascular access devices in hemodialysis or as an accessory to an I.V. set for the administration or withdraw of fluids to a patient through a cannula or needle placed in the vein or artery. The device may be used for patient populations including very low birth-weight infants, infants, children, and adults for up to 7 days
2 O-Grady, Naomi, et al. “Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011.” U.S. Centers for Disease Control and Prevention, 2011, pp. 19
3 Data on file
For complete indications, contraindications, warnings, precautions, and potential adverse events, refer to the product's Instructions for Use (IFU).
